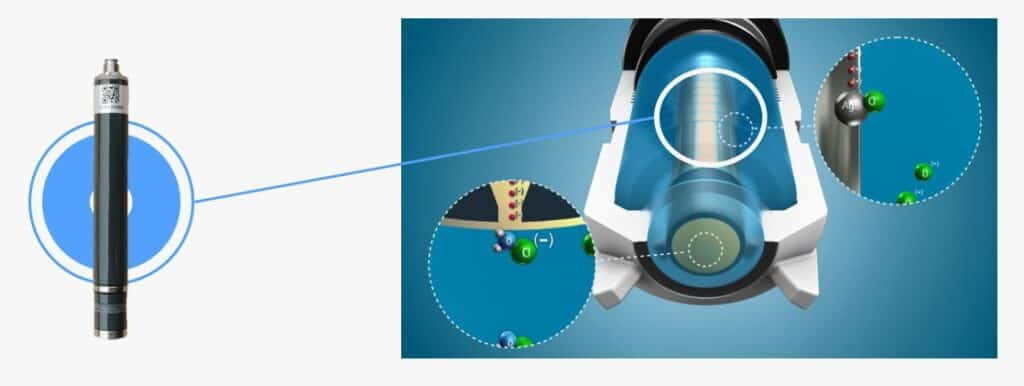
As we know, the free chlorine sensor is widely applied to online detection of free chlorine in drinking water, swimming pool, personal wellness, and so on. The detection process is to selectively pass the free chlorine molecules through the sensor to reach the surface of the cathode of the sensor. Under the action of the polarization potential, the free chlorine molecules generate The reduction reaction and anodic oxidation, then complete the entire redox reaction process. During this reaction process, it generates a current. The magnitude of the current is proportional to the free chlorine content, then we can calculate the free chlorine data by the magnitude of the current. Cut to the chase, the free chlorine sensor can be divided into a diaphragm polarographic free chlorine sensor and a constant voltage free chlorine sensor according to the measurement principle.
The principles of these two types of sensors are different and we will elaborate on them separately below.
The diaphragm polarographic free chlorine sensor consists of a cathode, an anode, an electrolyte, and a permeable membrane. The free chlorine molecules reach the surface of the cathode through the selective membrane of the sensor. After the polarization potential is polarized, the free chlorine molecules are reduced by electrons, and the anode loses electrons to oxidize. At the same time, a current signal proportional to the free chlorine concentration is generated. The free chlorine concentration should be read according to the magnitude of the process current signal.
The constant voltage-free chlorine sensor includes a measuring system composed of three electrodes: a working electrode, a counter electrode, and a reference electrode. After the sensor is put into the water body, the controller’s circuit supplies a constant voltage to the working electrode, the counter electrode. In addition, the reference electrode provides the reference potential required by the circuit. Driven by this voltage, the working electrode and the counter electrode will react with the free chlorine in the water to generate a signal. This electrochemical reaction process will consume the free chlorine in the water sample, and the magnitude of the generated signal is directly related to the concentration of free chlorine in the water. The controller calculates the corresponding free chlorine concentration according to the signal generated by the reaction. The current intensity is linear.
Then you may ask what are the essential differences between those two types of chlorine sensors.
A diaphragm polarographic sensor works by applying a constant voltage to a working electrode and a counter electrode, separated by a permeable membrane or diaphragm. As chlorine diffuses through the diaphragm and reacts with water at the working electrode, a current is generated that is proportional to the concentration of chlorine. This current is measured and converted into a reading of free chlorine concentration.
In contrast, a constant voltage sensor applies a constant voltage to a working electrode and a reference electrode, which are in contact with the water sample being measured. The current that flows between the electrodes is measured, and the reading is proportional to the concentration of free chlorine.
The diaphragm polarographic sensor is generally considered to be more accurate and reliable than the constant voltage sensor, especially in applications where the water contains high levels of interference or other chemicals. However, it is also more complex and requires more frequent maintenance, such as cleaning or replacing the diaphragm.
The constant voltage sensor is simpler and easier to maintain, making it a more cost-effective option for some applications. However, it may be less accurate and reliable in certain conditions, such as in highly variable water quality or where there are high levels of interference.
Now hope you have general pictures of those two types of chlorine sensors. For further information or questions, please feel free to contact us.